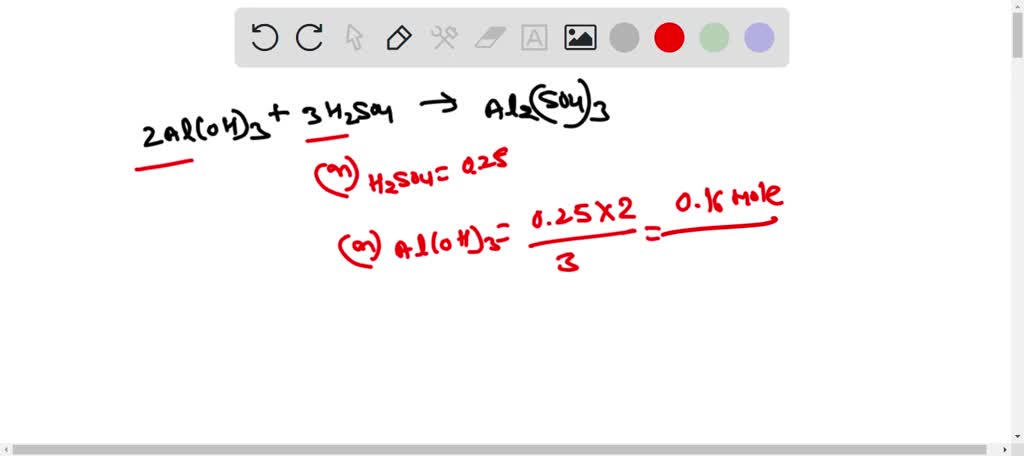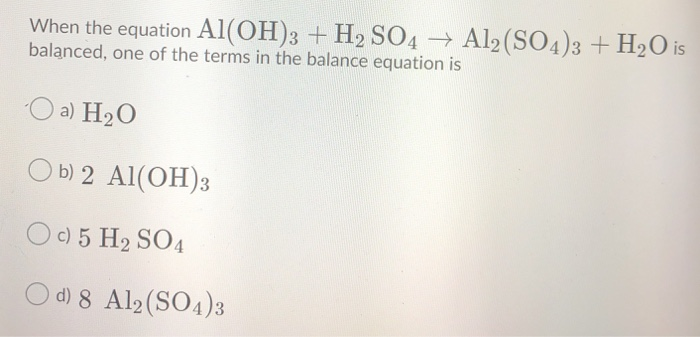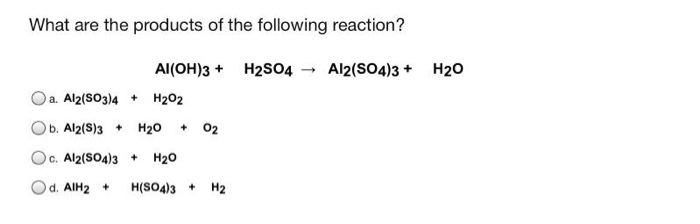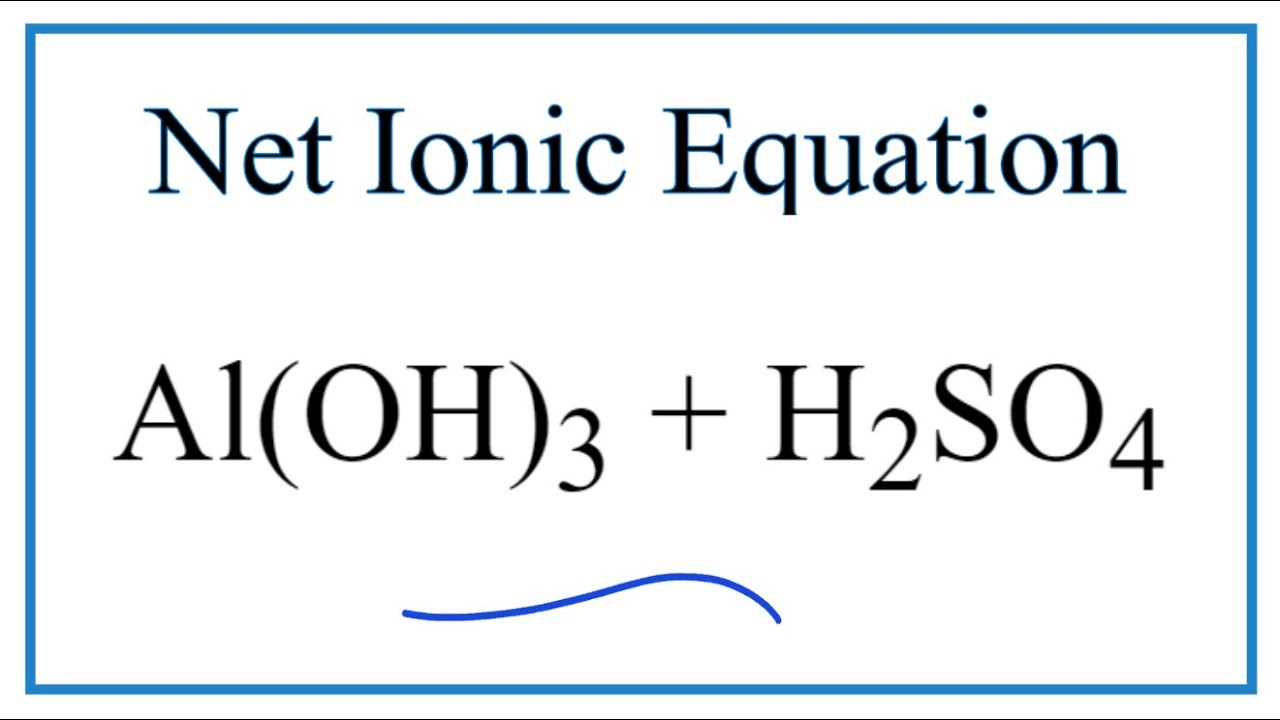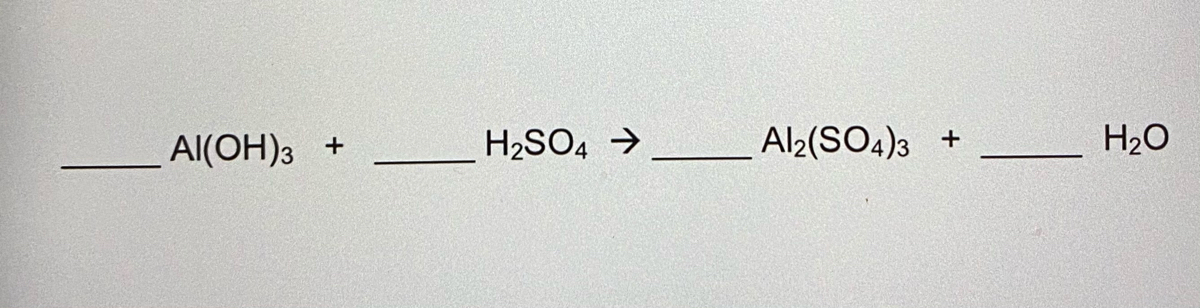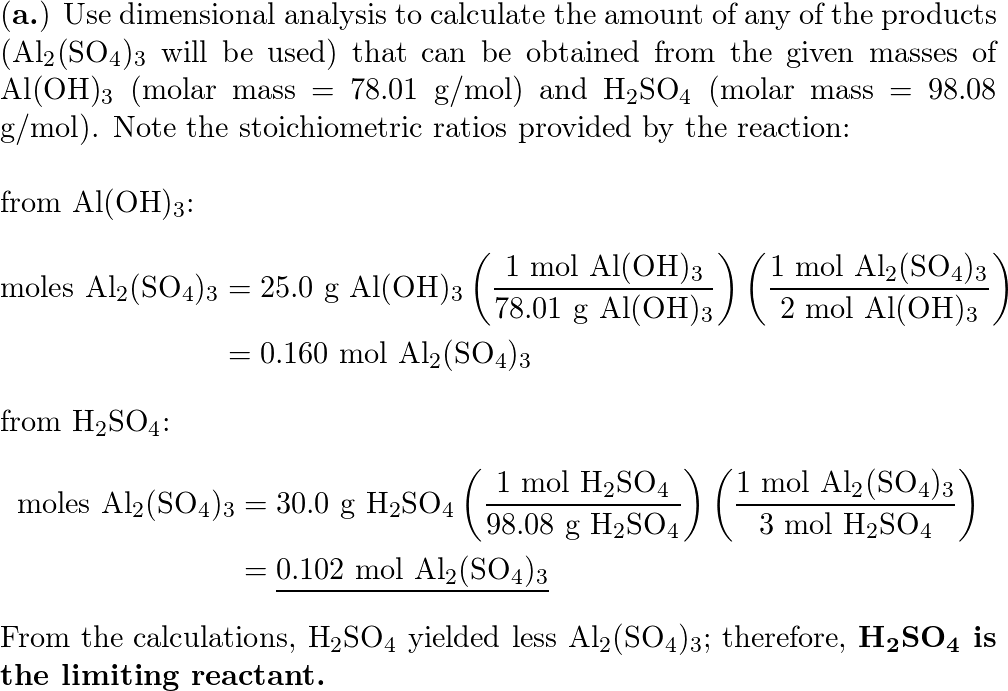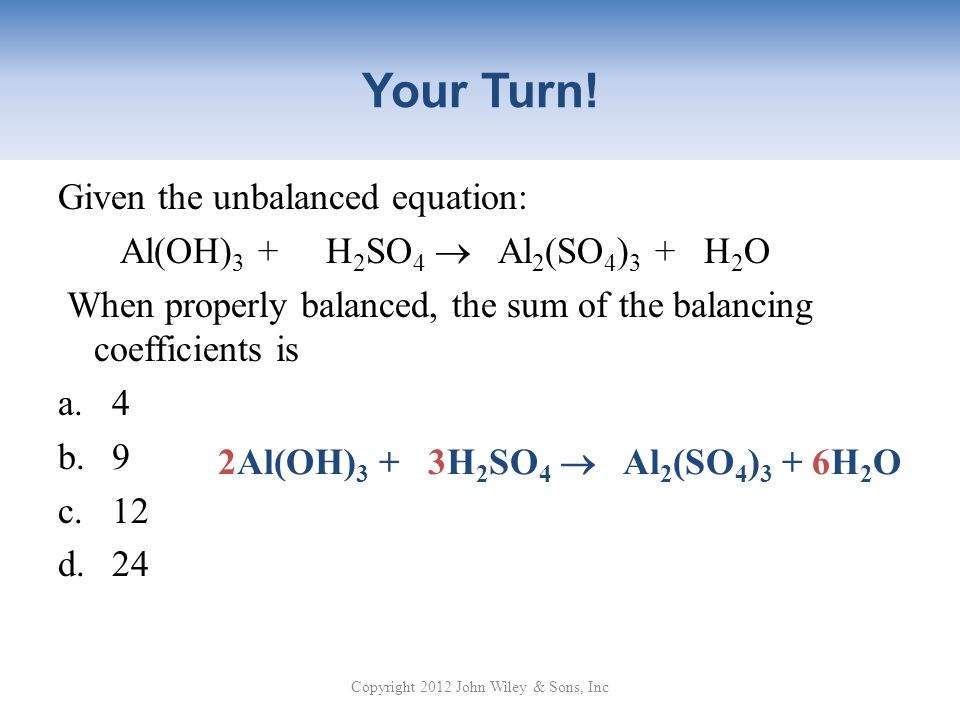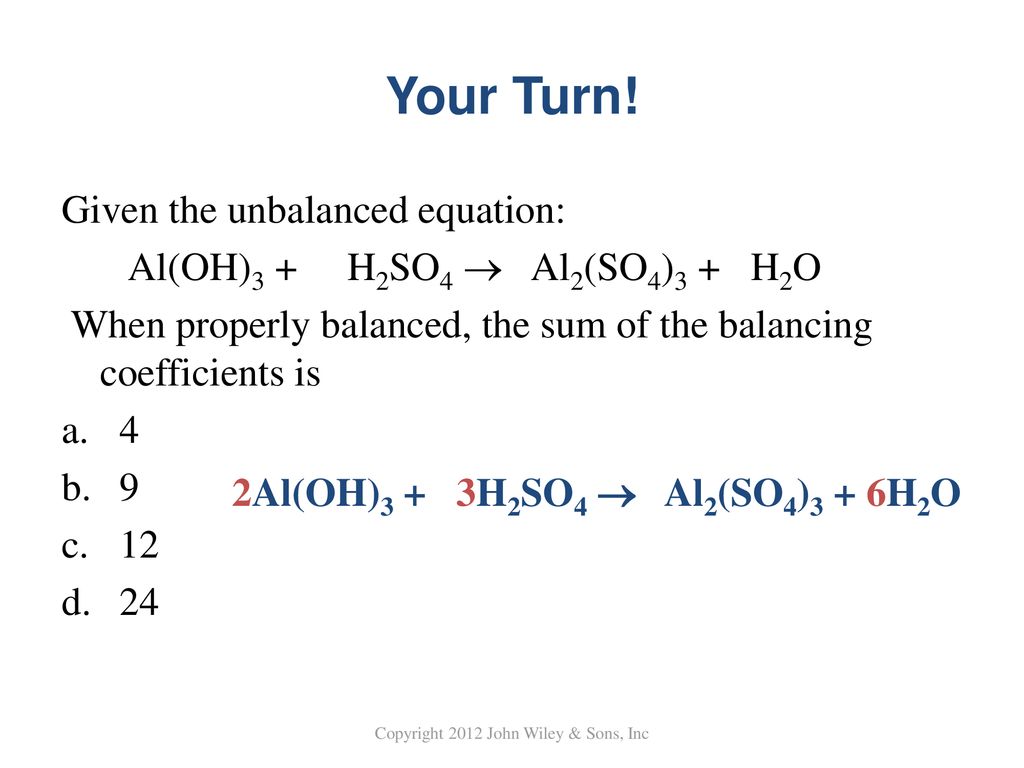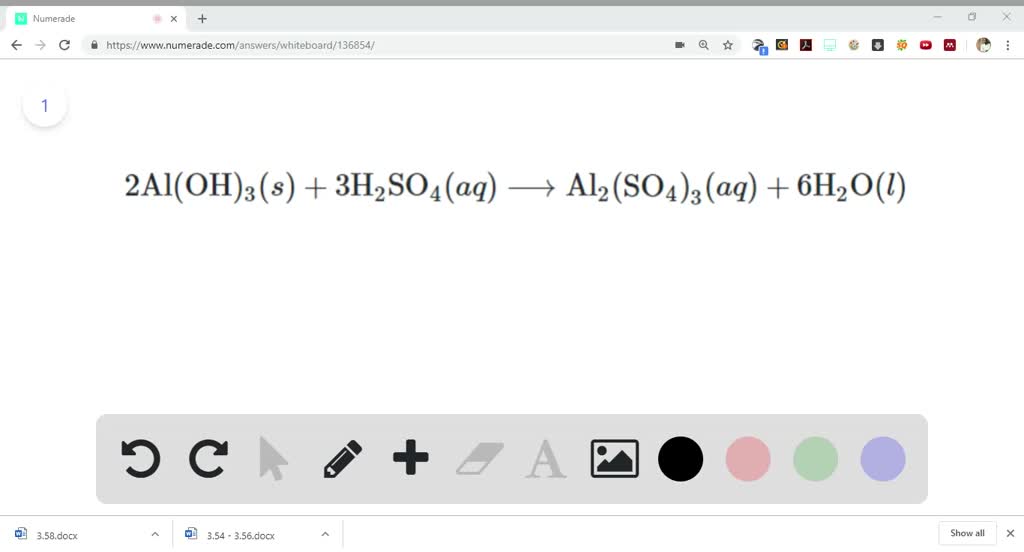
SOLVED:Aluminum hydroxide reacts with sulfuric acid as follows: 2 Al(OH)3 (s)+3 H2 SO4(a q) ⟶Al2(SO4)3(a q)+6 H2 O(l) Which is the limiting reactant when 0.500 mol Al(OH)3 and 0.500 mol H2 SO4 are

SOLVED: Aluminum hydroxide reacts with sulfuric acid as follows: 2 AI(OH)s(s) + 3 H,SO-(aq) Al,(SO4)s(aq) 6 H,O() Which is the limiting reactant when 0.500 mol Al(OH), and 0.500 mol H,SOa are allowed
How to balance this redox reaction using the oxidation number method? Al(s) + H2SO4(aq) → Al2(SO4) 3(aq) + H2(g) - Quora

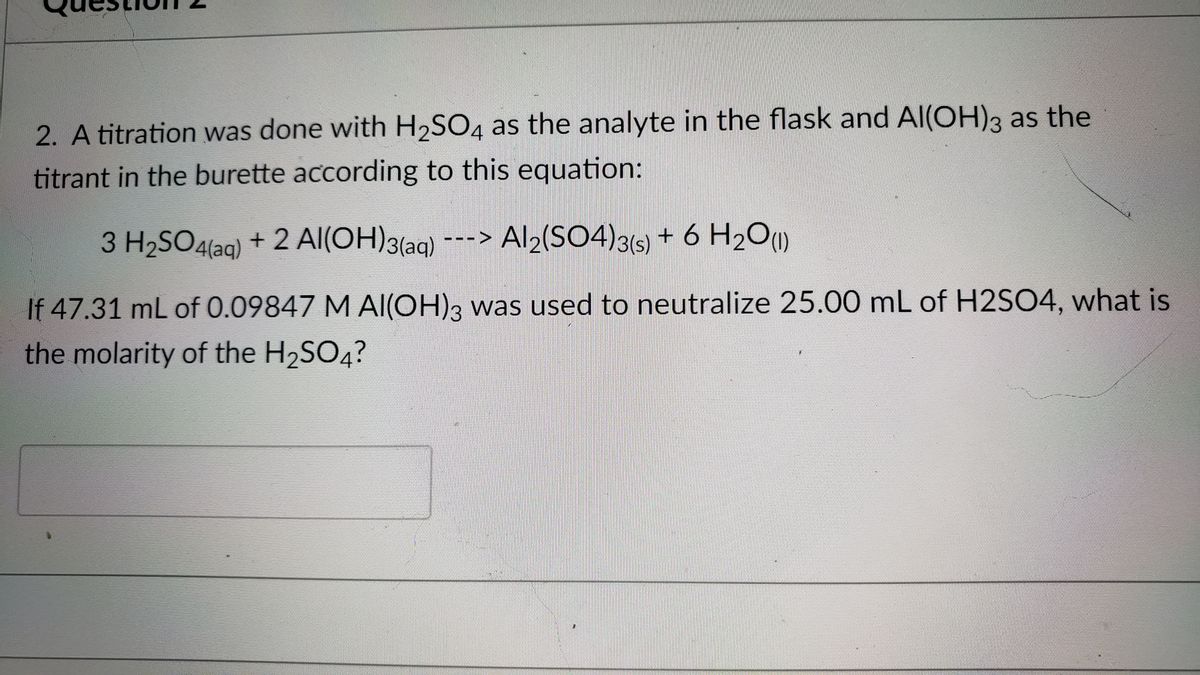
Alumminum hydroxide reacts with sulfuric acid as follows: 2Al(OH)3+H2SO4--> Al2(SO4)+6H2O. Which reagent is the limiting reactant when 0.500 mol Al(OH)3 and 0.500 mol H2SO4 are allowed to react? How ma | Homework.Study.com
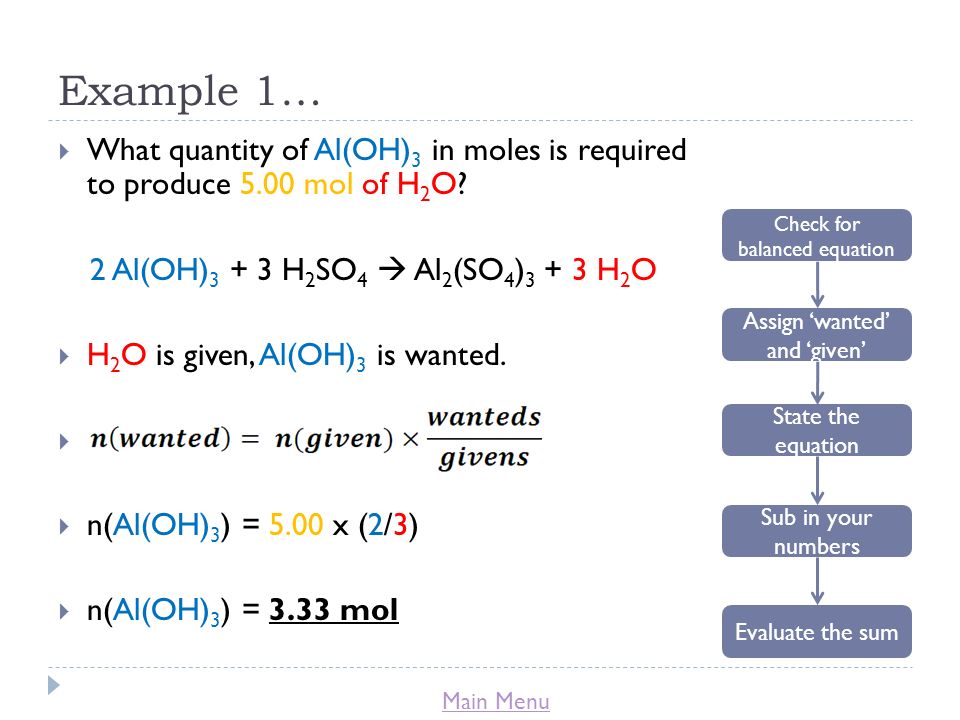
✓ Solved: When Al(OH)3 reacts with sulfuric acid, the following reaction occurs: 2Al(OH)3+3H2SO4→ Al2(SO4)3+6H2O...